Subproject B.6
Structure and Stability of Carbon Nanoclusters
How to deliberately contaminate diamond?
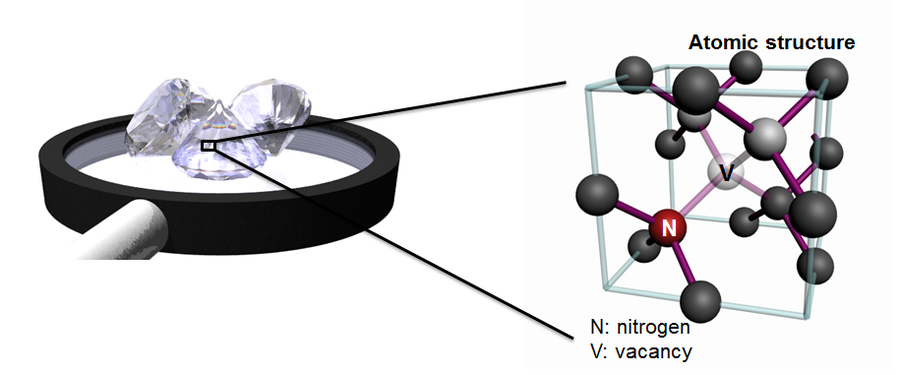
Carbon is found to build stable atom configurations in the form of graphite and diamond. Diamond is characterized amongst other things by its extreme hardness, electrical and thermal conductivity as well as its optical transparency. Deliberate contamination of diamond leads to defects with exceptional (photophysical) properties. These outstanding characteristics make these systems attractive for a broad range of applications. They can be used, for example, as qubits in future quantum computer hardware, as sensor material in ultra-sensitive measurements of magnetic and electric fields or as biomarkers, to allow monitoring cell processes. To achieve this it is necessary to produce very small nanodiamonds (diameter ~nm) containing stable structural and optical defects, e.g. nitrogen-vacancy centers (NV centers), or bulk diamond structures containing shallow defects with nanometer resolution (~10nm).
Using different simulation techniques (molecular dynamics (MD), Monte Carlo (MC), density functional theory (DFT), configuration interaction (CI)) it is possible to model and gain insight into important physical processes ranging from diamond fabrication to optical analysis, resulting in a detailed picture of relevant processes for the systems under investigation on the molecular level.
The purpose is to obtain a clear understanding of the physical properties of defects which are placed in the carbon matrix at varied distances from the surface. In particular the influence of surface termination on the optical properties of defects should be characterized and manipulated.
- Figure 1: Atomistic structure of the NV-center.
|
Further information about this subproject